
Solution
ZMI delivers full-spectrum OEM/ODM (OBL/Private Label) solutions, covering professional R&D, regulatory compliance, procurement, quality control, production, and after-sales support—ensuring your products consistently meet the highest standards.
Beyond manufacturing, ZMI provides expert guidance on international product registration and certification, helping clients efficiently navigate complex regulatory requirements. With extensive experience in Class I and Class II medical devices, ZMI is a trusted long-term partner for global brands seeking innovative, high-quality medical solutions.
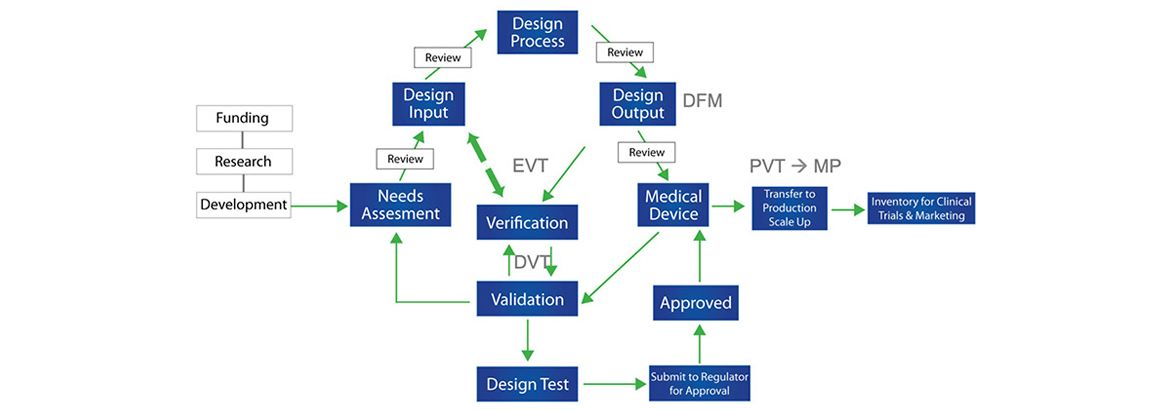
Medical Device Development Process
Physical Therapy
• Physical Therapy Medical DeviceSpinal Decompression (Traction)
Electrical Stimulation (TENS / NMES / IFC / Iontophoresis)
RF Diathermy (Ultrasound / Shortwave)
Light Therapy
Mobile TENS & EMS
Photobiomodulation Therapy (PBMT)
Functional Electrical Stimulator
Leadwires (Standard / Customized)
Electrodes (Reusable / Disposable)
Specialized Accessories
Biomedical Technology
• Orthopedics - DVT Prevention Management
• Otolaryngology - PEMF Tinnitus Treatment
• Dermatology - Cosmetic Accessories
• Healthcare - Virtual Reality & EMG feedback
• Life Science - Electroporation (Cell Fusion)
